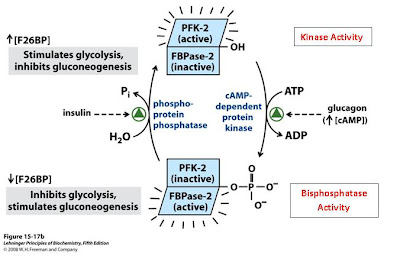
What does the bifunctional enzyme do?
Fructose 2,6-bisphosphate (F26BP) is an activator of glycolysis. This molecule increases the activity of the important rate-limiting enzyme phosphofructokinase-1 (PFK-1) by acting as an allosteric activator. The concentration of F26BP is directly linked to the activity of the unique bifunctional enzyme, more formally known as phosphofructokinase-2/fructose-2,6-bisphosphatase (PFK-2/FBPase), or PFKFB3. When the kinase activity is high, F26BP concentrations, and consequently glycolytic flux, increase. On the other hand, when the bisphosphatase activity is dominant, the concentration of F26BP decreases, removing the allosteric activator of PFK-1, and decreasing glycolytic flux (1,3).
How is the bifunctional enzyme relevant and applicable?
Glycolysis is an important process for cellular growth and research of its regulation, including the important bifunctional enzyme, has gained much attention. Cancer cell growth is attributed to high glycolytic flux and may be directly related to the bifunctional enzyme. Better understanding this enzyme may open up the door to cancer understanding and treatment. Recent research has shown that the bifunctional enzyme is directly affected by hypoxic conditions, which is the typical environment for neoplastic cells. Hypoxia activates both the bifunctional enzyme itself and HIF-1 (a transcription factor), which upregulates the expression of the bifunctional enzyme (4). Therefore, hypoxia produces more of the bifunctional enzyme and increases the activity of these enzymes. This obviously permits cancer cells to grow more quickly and rapidly. Additionally, it has been shown that ethylenediaminetetraacetic (EDTA) binds to the kinase active site of the bifunctional enzyme with high affinity. By blocking this site, F26BP cannot be made, inhibiting its activating effects on glycolysis (2). In conclusion, research marks the bifunctional enzyme as a potential for the treatment of cancer tumors (either directly or through the inhibition of HIF-1) and provides EDTA as a promising starting molecule for this treatment.
Sources:
- Bando H, Atsumi T, Nishio T, et al. Phosphorylation of the 6-Phosphofructo-2-Kinase/Fructose 2,6-Bisphosphatase/PFKFB3 Family of Glycolytic Regulators in Human Cancer. Clinical Cancer Research 2005; 11:5784-5792.
- Kim S, Manes N, El-Maghrabi R, Lee Y. Crystal Structure of the Hypoxia-inducible Form of 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3). Journal of Biochemistry 2006; 281:2939-2944.
- Chesney J. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis. Curr Opin Clin Nutr Metab Care 2006; 9(5):535-953.
- Bartrons R, Caro J. Hypoxia, glucose metabolism and the Warburg's effect. J Bioenerg Biomembr, 2007; 39(3):223-229.