Close your eyes and imagine this: it’s a Friday night and you’re sitting back, relaxing, reading a good ol’ biochemistry journal. The sun is coming down, the air is fresh…it’s a beautiful night. And then you come across it—phosphofructokinase-2/fructose-2,6-bisphosphatase (PFK-2/FBPase). Phosphofructowhaaat? Who gives something such a long name! Maybe such a long name would make sense if you were in a Hispanic Literature course reading a book by someone with a name like Jose Manuel Antonio Gutierrez Ortiz; sure, then a long name makes sense. But for an enzyme?
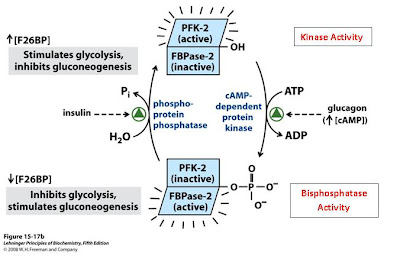
But that’s what makes this enzyme so great; the bigger name means more enzymatic activities! As you may recall, fructose 2,6-bisphosphate (F26BP) is an activator of glycolysis. It increases the activity of the rate-limiting enzyme phosphofructokinase-1 (PFK1) by acting as an allosteric activator. So how is this enzyme special? It is able to regulate PFK1 all by itself because it’s bifunctional! If you’re anything like me—a “one-track-minded” kind of person—you can quickly appreciate this. Think about those times when you’re studying hard and someone sits down to ask you a question. Or when you are typing away at the computer for hours and all of a sudden your mom calls. It’s so hard to just up and switch activities.
Thankfully for us, however, this is no problem for our friend PFK-2/FBPase. With a quick phosphoylation, this enzyme can change activity. One minute the enzyme is functioning as a kinase, phosphorylating F6P to F26BP to increase glycolytic flux, and the next its acting as a bisphosphatase, removing phosphates from F26BP to decrease glycolytic flux.
Kinase Activity
Bifunctionality of PFK-2/FBPase
So at your next meal remember to say thank you to our long-named enzymatic helper phosphofructokinase-2/fructose-2,6-bisphosphatase . Because while you have the luxury of focusing on that big, juicy, well-marinated steak, this enzyme is ready to respond to your body’s glycolytic needs.